Store
Store
Explore our Popular Assignments
MAGNETISM
In this chapter, Magnetism, we learn about magnets and their basic properties. We study natural and artificial magnets, their different forms, and the law of magnetic poles. The chapter explains the directive property of a magnet, which helps us understand why a freely suspended magnet always points in the North–South direction. We also learn about induced magnetism and magnetic induction, showing how magnetic materials acquire magnetism temporarily. The concept of a magnetic field is introduced through magnetic field lines, along with their important properties. Further, the chapter helps us understand the Earth’s magnetic field, including magnetic poles, the magnetic equator, and neutral points. Overall, this chapter builds a clear foundation of magnetism using simple experiments, diagrams, and observations.
Chemical equilibrum
Chemical Equilibrium Introduction Chemical equilibrium is a fundamental concept in chemistry that explains how reversible chemical reactions behave. Many chemical reactions do not go to completion; instead, they reach a state where reactants and products coexist. This condition is known as chemical equilibrium and is very important in understanding industrial processes, biological systems, and laboratory reactions. Meaning of Chemical Equilibrium Chemical equilibrium is the state of a reversible chemical reaction in which the rate of the forward reaction is equal to the rate of the backward (reverse) reaction, so that the concentrations of reactants and products remain constant with time. It is important to note that: Chemical equilibrium is dynamic, not static. Reactions continue to occur, but there is no net change in concentration. Reversible and Irreversible Reactions Reversible reactions are reactions that can proceed in both forward and backward directions. Represented using a double arrow (⇌) Example: Irreversible reactions proceed in one direction only. Represented using a single arrow (→) Example: Only reversible reactions can establish chemical equilibrium. Dynamic Nature of Chemical Equilibrium At equilibrium: The forward reaction continues converting reactants to products. The backward reaction continues converting products to reactants. The rates of both reactions are equal. Macroscopic properties such as colour, pressure, and concentration remain constant. Conditions Necessary for Chemical Equilibrium For chemical equilibrium to be established: The reaction must be reversible The reaction must occur in a closed system Suitable temperature and pressure conditions must be maintained Law of Chemical Equilibrium (Law of Mass Action) The law of mass action states that: At a constant temperature, the ratio of the product of the concentrations of the products to the product of the concentrations of the reactants, each raised to the power of their stoichiometric coefficients, is constant. This constant is known as the equilibrium constant (K). Equilibrium Constant (Kc and Kp) Kc (Concentration-based equilibrium constant) For a general reaction: The equilibrium constant expression is: Where: Square brackets [ ] represent molar concentration Solids and pure liquids are not included Kp (Pressure-based equilibrium constant) For gaseous reactions:
Infographics
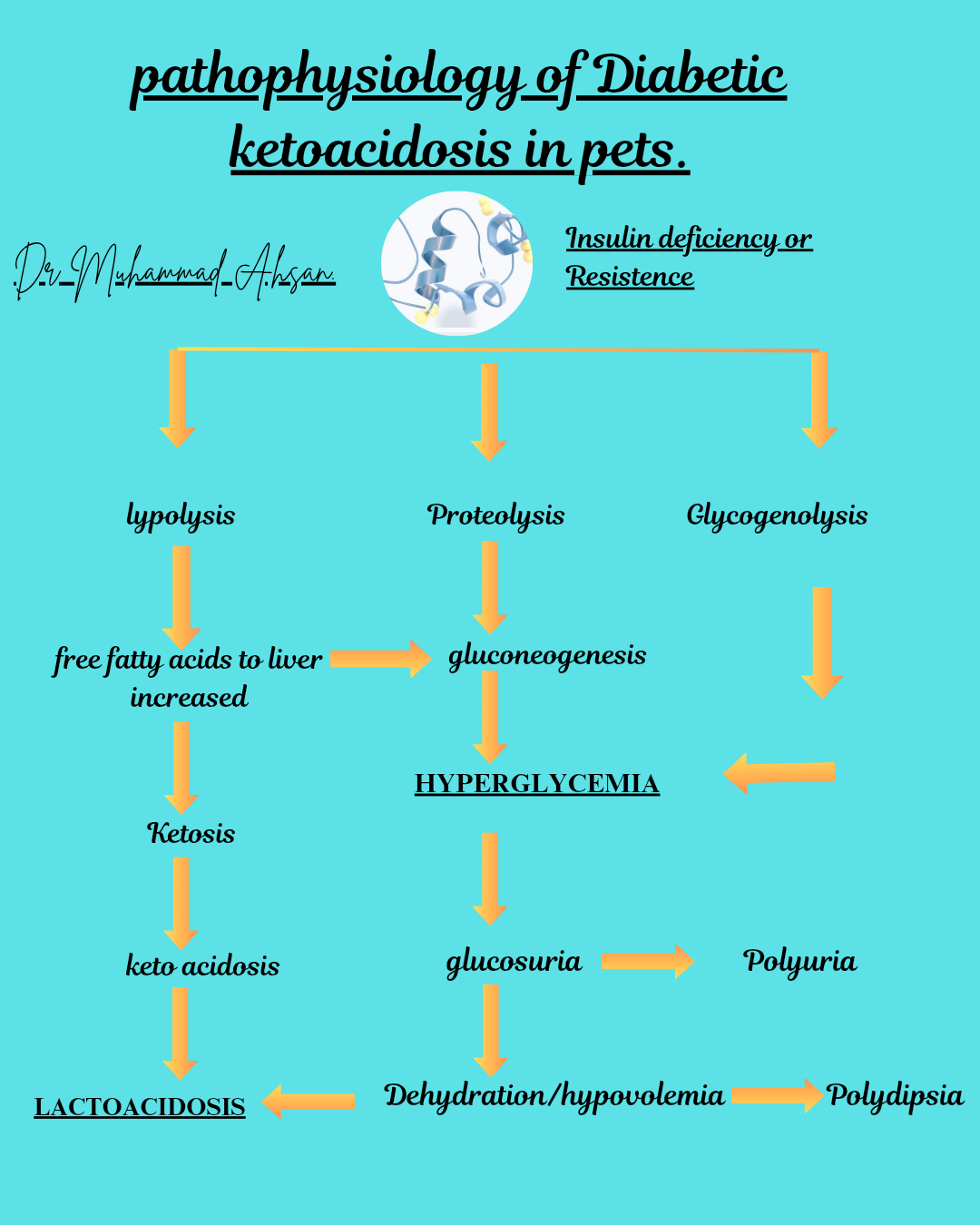
My notes are about brief detail of pharmacology of drugs commonly used in exotic animal.Each infographical post include use, mechanism of action, adverse effect drug interaction and doses.
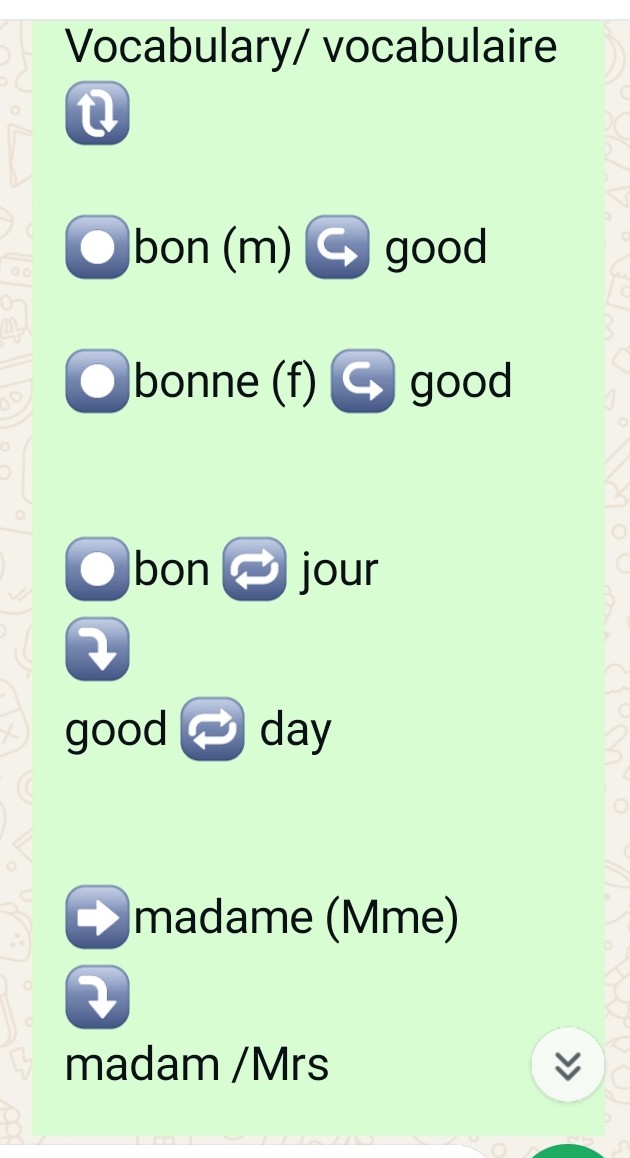
Beginners french
Vocabulary/ vocabulaire 🔃 âºï¸bon (m) â†ªï¸ good âºï¸bonne (f) â†ªï¸ good âºï¸bon 🔠jour â¤µï¸ good 🔠day âž¡ï¸madame (Mme) â¤µï¸ madam /Mrs âï¸mademoiselle (Mlle) â¤µï¸ Miss âºï¸monsieur (m.) â¤µï¸ Mr./Sir âºï¸Salut/salute ⤵ï¸hi bonsoir↪ï¸good evening â¤µï¸ âºï¸bon â†ªï¸ soir    good â†ªï¸ evening
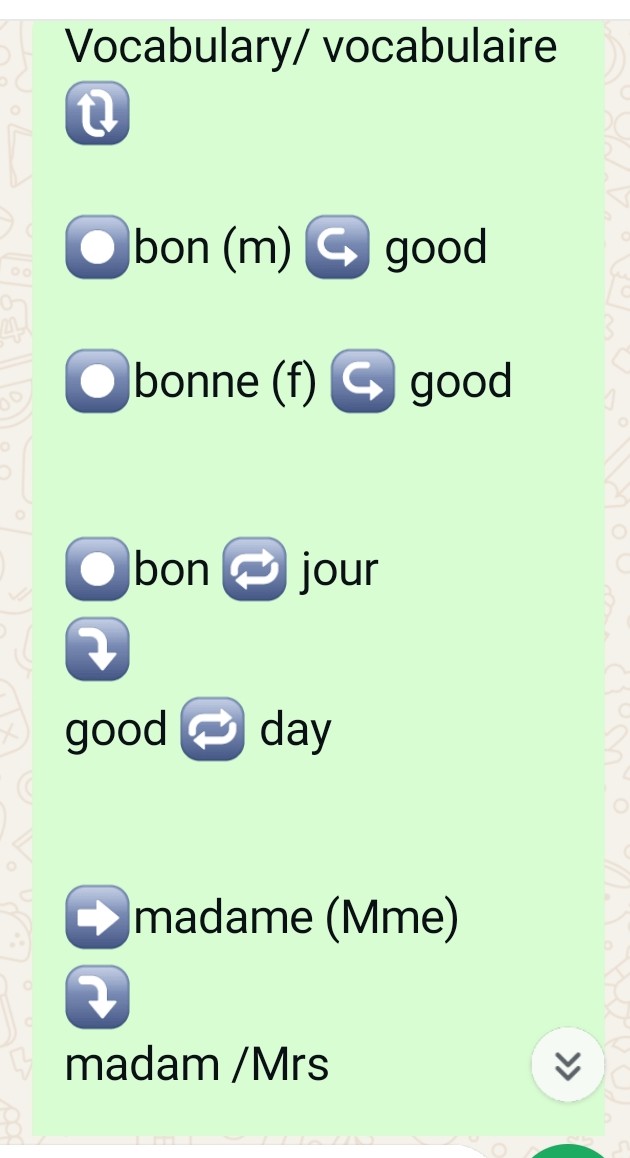
Beginners french
Vocabulary/ vocabulaire 🔃 âºï¸bon (m) â†ªï¸ good âºï¸bonne (f) â†ªï¸ good âºï¸bon 🔠jour â¤µï¸ good 🔠day âž¡ï¸madame (Mme) â¤µï¸ madam /Mrs âï¸mademoiselle (Mlle) â¤µï¸ Miss âºï¸monsieur (m.) â¤µï¸ Mr./Sir âºï¸Salut/salute ⤵ï¸hi bonsoir↪ï¸good evening â¤µï¸ âºï¸bon â†ªï¸ soir    good â†ªï¸ evening
English for Young Learners
These lessons make learning basic vocabulary and simple expressions fun, even for absolute beginners, while colorful story-based lessons let more confident learners see how language is used in everyday life.
English Conversation
These lessons cover a variety of topics to help beginner to advanced students improve their communication skills.
Business English
These lessons cover the essential language skills needed to communicate clearly in an English-speaking professional environment. Students can practice vocabulary and expressions through a variety of exercises, including listening activities and role plays.
Picture, song or guessing, , role play or imitation
Warm up IMAGE : use of image related to the current lesson and through the image I teach the vocabulary. Image in which people are acting accordingly to the sentence i want to teach . ROLE PLAY : set a dialogue between students if it is group. If it is one learner, the teacher initiate a conversation between the learner and I ( the teacher ). IMITATION imitate the sounds of animals or thing and the student says the name of that animal or that thing. SONG or Guessing : teacher create song with the new vocabulary words. Then, i make the student sings with me during 5 min. FOR INTERMEDIATE : use Authentic text or guessing GOAL : student practice a lot speaking by describing everything around them accordingly to the lesson
Picture, song or guessing, , role play or imitation
Warm up IMAGE : use of image related to the current lesson and through the image I teach the vocabulary. Image in which people are acting accordingly to the sentence i want to teach . ROLE PLAY : set a dialogue between students if it is group. If it is one learner, the teacher initiate a conversation between the learner and I ( the teacher ). IMITATION imitate the sounds of animals or thing and the student says the name of that animal or that thing. SONG or Guessing : teacher create song with the new vocabulary words. Then, i make the student sings with me during 5 min. FOR INTERMEDIATE : use Authentic text or guessing GOAL : student practice a lot speaking by describing everything around them accordingly to the lesson